The Cost of Mental Effort: Brain Energy in Health and Disease
The ability to fuel cognition, and the failure to due so in disease, is of central importance to brain function. Metabolic neuroscience is a field on the frontier of innovation.
Innovation in the mid-19th century was wild. It was a time of rapid industrialization, where the clang of steam engines and the hiss of factories marked progress, and the scent of coal smoke hung heavy in the air. It was an era when candlelight still flickered in homes but gas lamps were beginning to illuminate city streets, casting long shadows on cobblestone roads.
Amid this backdrop, scientific revelations were reshaping human understanding, and one of the most profound was the Law of Conservation of Energy. Established by pioneers like Julius Robert von Mayer and James Prescott Joule, this principle stated that energy could neither be created nor destroyed, only transformed. While its implications for physics and engineering were clear, it also stirred debates in psychology.
Could the act of thinking involve a unique form of energy?
E. L. Youmans (1821–1887) argued that mental operations depended on material changes in the nervous system, linking thought to physical processes. Kurd Lasswitz (1848–1910) later introduced the concept of “psychophysical energy,” proposing that brain’s electrical activity connected the physical and mental realms.
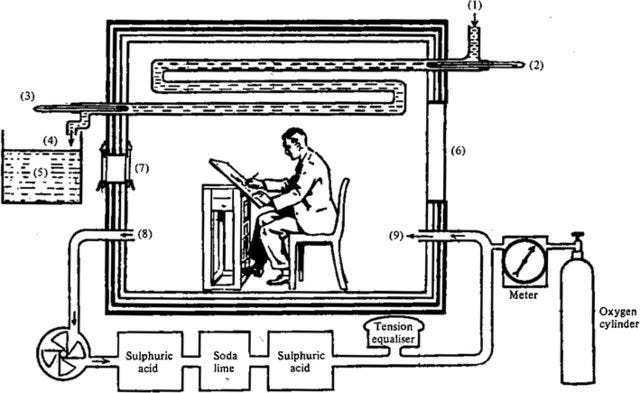
By the early 20th century, Alfred Lehmann (1858–1921) claimed that intense mental effort increased oxygen utilization, suggesting evidence for a specific psychic energy—a view Hans Berger (1873–1941) would build upon with his pioneering work on brain waves (the inventor of EEG). However, F. G. Benedict (1870–1957), with his precise respiration calorimetry experiments, concluded that mental effort had minimal effect on brain metabolism, challenging the notion of a distinct psychic energy.
Benedict was measuring the amount of oxygen consumed during intense cognitive tasks, determining that mentally exhausting work did not measurably change the amount of energy required. Meaning, mental effort does not increase the amount of calories burned.
These early debates laid the foundation for future studies in cognitive neuroscience, probing how the mind transforms energy into thought and perception. (Read more here)
The Brain as a Model of Energy Efficiency
How much energy does biology’s most energetically efficient and sophisticated computer—the human brain—require? Despite its significant demands, Benedict’s conclusions remain true today… cognitive function requires remarkably little energy.
The brain operates on approximately 20 watts of power—comparable to the energy consumption of a dim light bulb—yet it can perform an astonishing array of complex tasks. These include pattern recognition, decision-making, learning, language processing, and controlling bodily functions, all in real-time.
The brain’s efficiency arises from its highly specialized and parallel architecture. Neurons communicate through both electrical and chemical signals, enabling efficient processing and transmission of information. Additionally, the brain adapts and reorganizes its neural pathways through a process called neuroplasticity, optimizing its operations based on experience and learning.
Our Understanding of Brain Metabolism is Limited by Available Tools
Much stated above is rooted in theory, modeling, and attempts of computation to mimic neural architecture.
Current high throughput methods for characterizing the brain in states of health and disease rely heavily on available, scalable methods— namely genome sequencing and bulk and single cell RNA sequencing. However, static measures of the genome, or even quantitative expression of mRNA, do not correlate well with energy expenditure or metabolic flux (e.g. changes in mRNA content of Pyruvate Dehydrogenase Complex Enzymes may or may not correlate with the pyruvate conversion to acetyl CoA— the key link between glycolysis and the tricarboxylic acid (TCA) cycle AKA the Krebs Cycle).
For this reason, a deep understanding of metabolic function of the brain is lacking. Due to over-reliance on scientific methods of convenience, we are in the infancy of unlocking the potential of metabolic neuroscience. We humans appear limited in understanding things we can’t see. We can’t comprehend the impact of neural metabolism if we don’t use the tools to reveal it.
A Mechanistic Understanding of Brain Energy Metabolism
How does the brain manage to keep energy consumption so low despite the perception of enormous mental effort? The answer lies in a series of finely tuned mechanisms that optimize metabolic efficiency:
Key Metabolic Pathways (Hint: Its not only mitochondria)
Cytoplasmic Energy Metabolism (e.g. outside of the mitochondria, in the cell)
Glycogen Metabolism & Glycolysis
Glycogen stored in astrocytes plays a crucial role in short-term energy buffering during periods of intense cognitive activity. Studies by Doug Rothman’s theoretical and experimental group at Yale have illuminated the significance of glycogen in maintaining energy supply for neuronal function.
Glycolysis happens outside of the mitochondria, and is the conversion of glucose to pyruvate. This yields ATP in a process that is >10x less efficient than complete metabolism of glucose in the mitochondria, but can occur when energy demand outpaces oxygen consumption.
Creatine Phosphate System
The brain is second only to muscle tissue in its storage of creatine phosphate, which serves as a rapid reserve for ATP synthesis. This mechanism allows for the quick restoration of ATP during brief, high-demand periods.
Creatine phosphate can pass its phosphate molecule to ADP (adenosine Di-phosphate) to make ATP (adenosine Tri-phosphate). This allows for ATP production very quickly, and does not rely on the mitochondria. However this mechanism is only possible for very short bursts of energy due to limitations in creatine concentration and phosphate availability.
Mitochondrial Dependent Metabolism
Mitochondrial Metabolism – Oxygen-Dependent Glucose and Ketone Metabolism
The mitochondria are pivotal in energy production, converting glucose and ketones into ATP in an oxygen-dependent manner. This pathway’s efficiency is crucial for sustaining cognitive processes and is a determinant of maximal ATP synthesis rates.
Both glucose and ketones can contribute carbons to the TCA cycle, which allows for the efficient generation of ATP.
Neuron-Glial Crosstalk and the Glutamate-Glutamine Cycle
The interplay between neurons and glial cells supports synaptic function and neurotransmitter recycling. The glutamate-glutamine cycle, involving astrocytes and neurons, is central to managing excitatory signaling and energy use.
Interestingly, all of these pathways converge on the production and usage of ATP, the universal energy currency.
I believe that the most important metric of cellular health is the maximal rate of ATP production. This is because it is an integrated metric of the capacity to respond to any energetic stress that may be imposed on us.
Defects in Metabolism Can Lead to Brain Disease
When certain metabolic pathways are disrupted, they can manifest in various neuropsychiatric conditions:
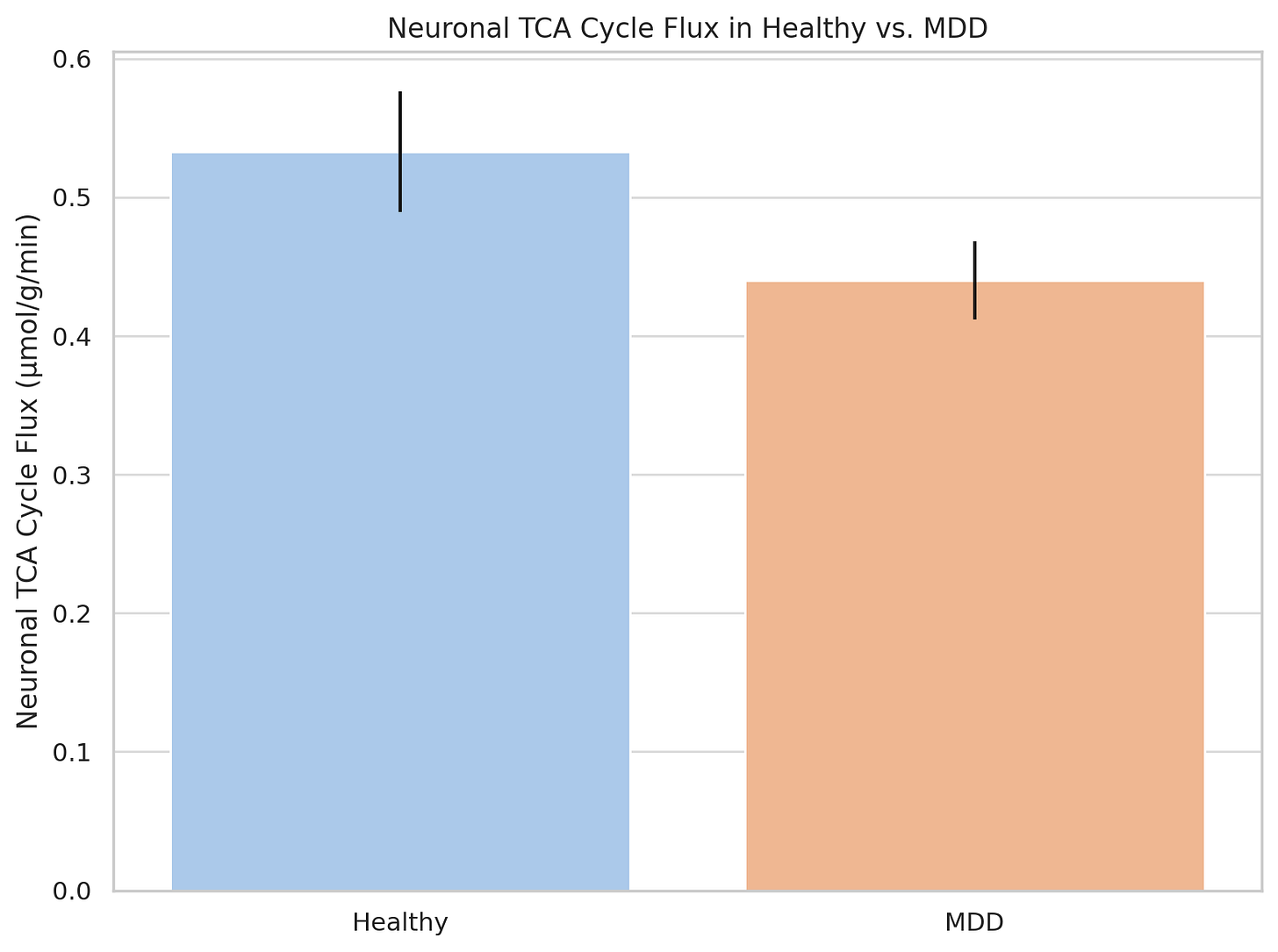
Depression: Human studies have demonstrated that TCA cycle flux rates (meaning the ability to generate energy via this mitochondrial pathway) is impaired in the brain of patients with major depressive disorder.
Neurodegeneration: Alzheimer’s disease is characterized by a decline in maximal ATP synthesis, contributing to impaired cognitive and motor function.
Epilepsy: Disruptions in the glutamate-glutamine cycle can lead to metabolic disarray. For instance, certain anti-seizure medication combinations (e.g. valproic acid and topiramate) can trigger hyperammonemia, leading to neuropsychiatric symptoms or even coma.
Inborn Errors of Metabolism: Genetic defects in genes related to metabolism have even been discovered and diagnosed due to strange changes in patient behavior, demonstrating the intrinsic link between genetics, metabolism, and the mind.
Solutions Needed
To innovate beyond current treatments, we need:
Functional Assays to Guide Diagnostics and Treatments: High-throughput tests to measure maximal ATP synthesis rates for more precise diagnostics and treatment monitoring. If improving function matters to you, rather than applying a bandaid, this is how to do it.
Metabolic-Targeted Interventions: Therapies aimed at increasing ATP synthetic capacity, potentially through:
Gene Therapy: To enhance mitochondrial function or correct metabolic deficiencies. See this review on mitochondrial gene therapy. An interesting starting point could also be the correction of inborn errors of metabolism characterized by neuropsychiatric behavior changes.
Bioelectronic medicine: Transcranial magnetic stimulation, focused ultrasound, and other methods of neuron-targeted electrical stimulation can challenge specific brain regions to improve energy metabolism.
Mitochondrial Uncouplers: By challenging the mitochondria to keep up with the “uncoupling” of electron transport from ATP synthesis, you can train your mitochondria to up-regulate ATP synthetic machinery. This is exercise for your neural mitochondria.
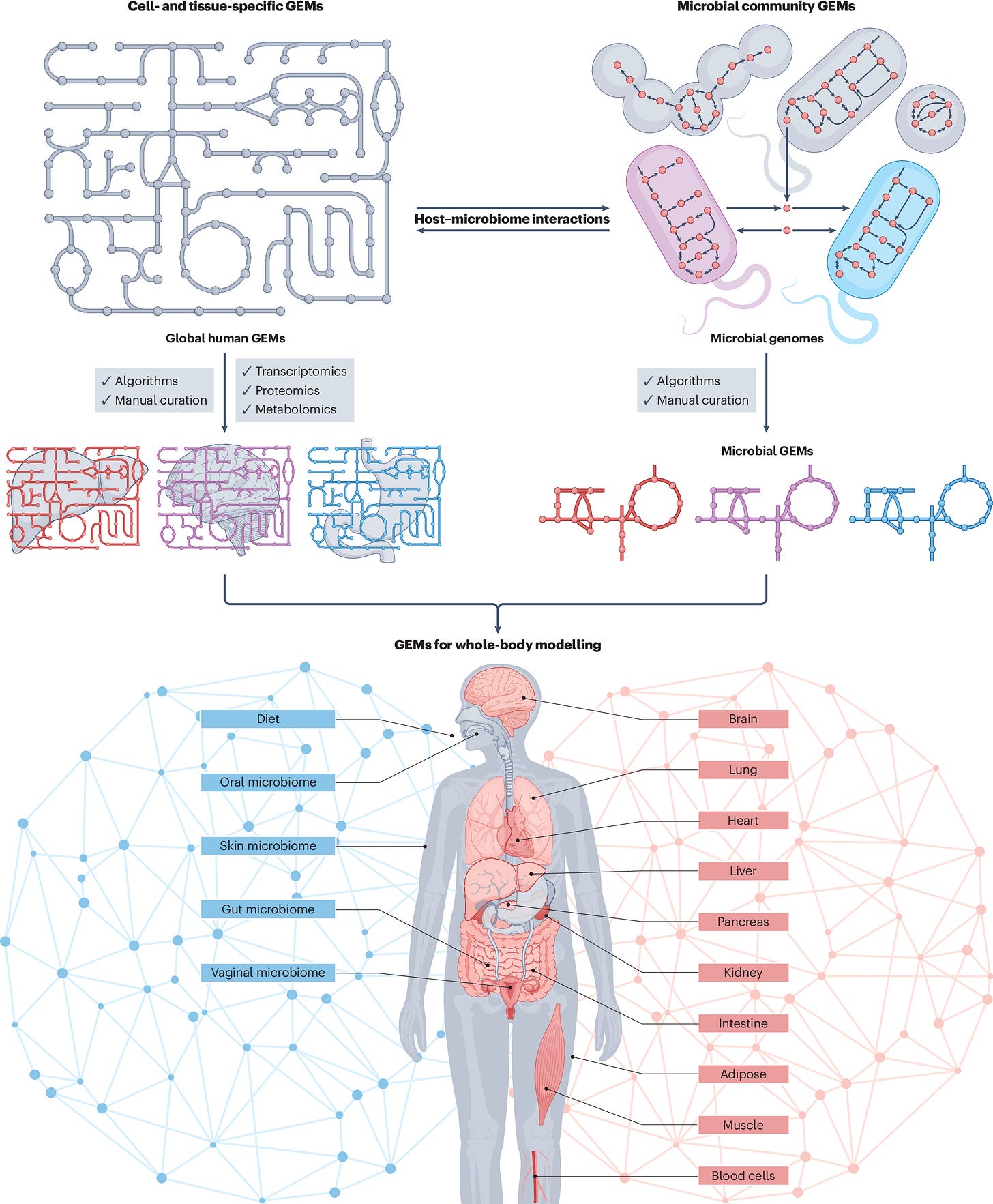
Genome Scale Metabolic Models (GEMMs) of the Human Brian
Imagine if we could model the interaction between every single microbe within or on us, and all of the different tissues within us. The unique genomic make up of all of us may impact the complex interplay between the metabolites the gut microbes can produce and the molecules that activate neuronal receptors. With GEMMs, we can do this. We can simulate gene knockouts, changes in the microbiome, alterations of diets, and how this ultimately influences brain function.